A) sodium oxide, Zinc oxide , Magnesium oxide
B) Aluminium oxide, Calcium oxide, Zinc oxide
C) Potassium oxide, Lithium oxide, Carbon monoxide
D) Silver oxide, Lead oxide, Sodium oxide
E) Sulphur dioxide, Aluminium oxide, Carbon monoxide
Show Answer
The correct answer is B .
A) sulphur (VI) oxide
B) hydrogen chloride
C) sulphur (VI) oxide
D) hydrogen sulphide
Show Answer
The correct answer is D .
The contact process is used for the industrial production of
Options:A) sulfuric acid (H2SO4)
B) Hydrochloric acid (HCl)
C) Sodium hydroxide (NaOH)
D) Calcium oxide (CaO)
Show Answer
The correct answer is A .
A) In making bicycle chains
B) As abrasives
C) In cutting glass and metals
D) As dies for drawing wires
Show Answer
The correct answer is A .
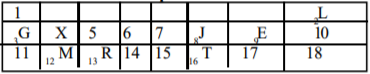
Use the section of the periodic table above to answer this question.
Which letter represents a non-metal that is a solid at room temperature?
Options:A) T
B) R
C) J
D) X
Show Answer
The correct answer is A .
A) undergoes reduction
B) serves as the positive electrode
C) production electons
D) uses up electrons
Show Answer
The correct answer is C .
A) 2.50
B) 3.50
C) 3.75
D) 7.50
Show Answer
The correct answer is C .
A chemical widely used as a fertilizer is?
Options:A) galena
B) bauxite
C) emerald
D) nitrochalk
Show Answer
The correct answer is D .
A) Make it more malleable
B) Remove the impurities unit
C) Protect it against corrosion
D) Render it passive
Show Answer
The correct answer is C .
The best treatment for a student who accidentally poured conc tetraoxosulphate(vi) on his skin in the laboratory is to wash his skin with?
Options:A) with cool running water
B) sodium hydroxide solution
C) iodine solution
D) sodium trioxonitrate(v) solution
Show Answer
The correct answer is A .